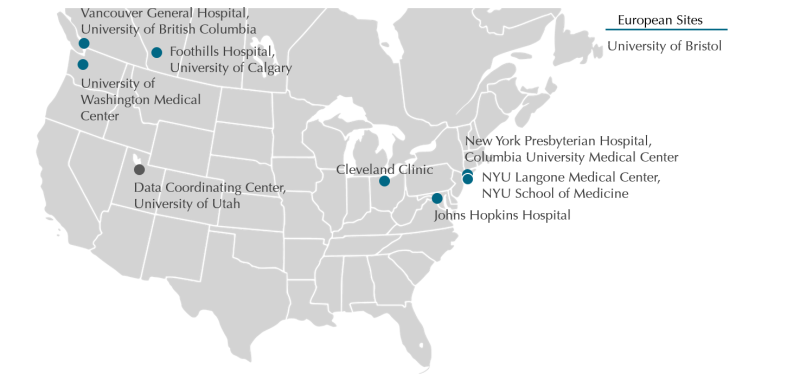
The AHCRN is composed of 8 clinical sites in the US, Canada, and Europe
Primary Investigator: Thomas Zwimpfer, MD, PhD, FRCSC
Location: 2329 West Mall, Vancouver, BC, Canada V6T 1Z4
Primary Investigator: Mark Hamilton, MDCM, FRCSC, FAANS
Location: 1403 29 Street NW, Calgary, AB, Canada T2N 2T9
Principal Investigator: Michael Williams, MD, FAAN
Location: 1959 NE Pacific St, Seattle, WA 98195
Principal Investigators: Sean Nagel, MD and James Liao, MD, PhD
Location: 9500 Euclid Ave, Cleveland, OH 44195
Principal Investigators: Abhay Moghekar, MBBS and Mark Luciano, MD, PhD
Location: 1800 Orleans St, Baltimore, MD 21287
Principal Investigators: Jeffrey Wisoff, MD and James Golomb, MD
Location: 650 W 168th Street, New York, NY 10032
Principal Investigator: Guy McKhann II, MD
Location: 333 E 38th Street, New York, NY 10016
Principal Investigator: Richard Edwards, MD, FRCS
Location: Senate House, Tyndall Ave, Bristol B28 1TH, United Kingdom
The AHCRN is currently enrolling patients!
Registry Eligibility:
- All Transitioning Hydrocephalus Patients
- All Arrested Hydrocephalus Patients
- New Acquired Hydrocephalus Patients
- New Normal Pressure Hydrocephalus Patients

For Researchers
The goal of the AHCRN is to be an open network engaged in concurrent multi-center clinical trials. Currently, the AHCRN is composed of 5 clinical sites, a data coordinating center, a cerebrospinal fluid (CSF) biobank, and an image depository. At this early juncture, the ACHRN is closed to additional sites.
If you would like more information regarding the AHCRN please contact:
Chair AHCRN
Mark Hamilton, MDCM, FRCSC
Department of Clinical Neurosciences
University of Calgary, Foothills Hospital
Calgary, Alberta, Canada
Email: mghamilton.hydro@gmail.com
CSF Biobank
Mark Luciano, MD, PhD
AHCRN Principal Investigator
Johns Hopkins Medical Center
Baltimore, MD
Email: markluciano@jhu.edu
